Keeping a houseplant or potted plant alive is good. Keeping a plant healthy with shiny leaves and multiple blooms is better. Using the right fertilizers is paramount to success. But the fertilizer choices are so confusing! Here are the highlights and uses of fertilizers and soil amendments with two specific things to remember.
What is Fertilizer?
Plant food actually comes in the form of light, captured by plants and converted to carbohydrates through photosynthesis. The sun provides the energy the plants need and stores it within the plant. Energy is food.
But the molecules containing elements plants need to bloom (P & K), form strong cell walls (Ca), develop healthy roots (P) or a dark green colour (N & S) are sometimes deficient and must be added as fertilizer. If you are growing a plant indoors or in a confined pot or planter there is a good chance your plant needs fertilizer.

Spinach in my greenhouse this November is glossy and green even though I am not heating the greenhouse yet and evening temperatures have been down to minus 10. Is this because of the greenhouse, the wise use of fertilizer or just good luck? It’s a combination I guess! Fertilizers can make plants more cold-hardy and the glossy leaves suggest these plants are fertilized to perfection.
Fertilizers help build a heathy, not just living plant. I used to be in the tough love group of gardeners adding zero or limited fertilizer and then I realized my plants could be so much stronger, bigger and more bountiful if I went to the dark side of adding supplements or at least commercial fertilizers to my house plants and potted plants.*
(*The topic of fertilizers for outdoor plants is so much more complicated because our soil often supplies the needed elements, as it did in my early gardens in Calgary. Even though our garden soils around the world vary so much I have normally subscribed to the “feed the soil, not the plant” theory of outdoor plant growth in my rich clay soil in Alberta, Canada. In my sandy soil on Vancouver Island I was humbled and became a heavy user of organic matter plus slow release fertilizer to get the plants up to my Alberta standards.)

A fertilizer lists the main nutrients it will provide for plant growth but it is not a source of energy. Energy comes from the sun or from grow lights. This is why if a plant tolerates shade, like lettuce, it can be grown in a partially shady site. If it needs full sun, like tomatoes, give it at least 4-5 hours of direct sun or light.
If your plant can separate out the elements in fertilizer from the complex package it is offered so much the better. If it is tough for the plant to access the actual available ion (or charged particle) because of some complexity in your soil or water or other nutrients offered – you will get very slow or zero response and the plants will look bad as they do in the top photo. See the Khan Academy for excellent videos about what is an ION.
An eggshell , for instance, is a possible source of Calcium. Bonemeal, for instance, is a possible source of phosphorus. But a water soluble fertilizer labelled as containing either Ca or P is probably more readily to plants. Water soluble fertilizers are designed to go right to work, providing the plant with the needed item. If you grow in a largely organic or low clay soil you will need to help the plants in your care by fertilizing them, not just adding eggshells or bonemeal.

Egg shells, such as these shown in a museum in Quebec can take a long time to break down and become available to plants. These eggshells are from the 17th century and they still look like egg shells. Chances are if they were still in the ground they would not be available to plants yet.
In my podcast “Helping Gardener’s Grow” #17, Dr. Chris Trobacher, research scientist for a fertilizer manufacturer in Canada, told us why fertilizer works and how to make sure you buy a source of fertilizer that works more effectively for your plants. Listen to the podcast HERE.
In an earlier podcast and in his book, Steve Solomon (author of The Intelligent Gardener) told us how to get “vibrating vegetables” by combining your own nutrient sources such as seed meal, feather meal, kelp, blood meal, fish bone meal and more. Warning: I love Steve and his information but I had to reduce our 3 hour interview greatly because it wandered off topic frequently and there were sound issues because Steve’s house in Tasmania, Australia was being renovated as we spoke. Consequently, unless you read his book you will likely miss a lot of the details in our interview. Also, like egg shells, I suspect many of the items used in Steve’s recipes take ages to break down and become available to plants unless you also have a vibrant living soil full of hard working micro-organisms. Steve’s recipes rely on a biologically active natural soil and are not going to work as well in a naturally lean sandy soil or for houseplants in “sterile” potting mix.
One more complication is that the different nutrients plants used can cancel or reduce other nutrient as seen in the mulders chart below.
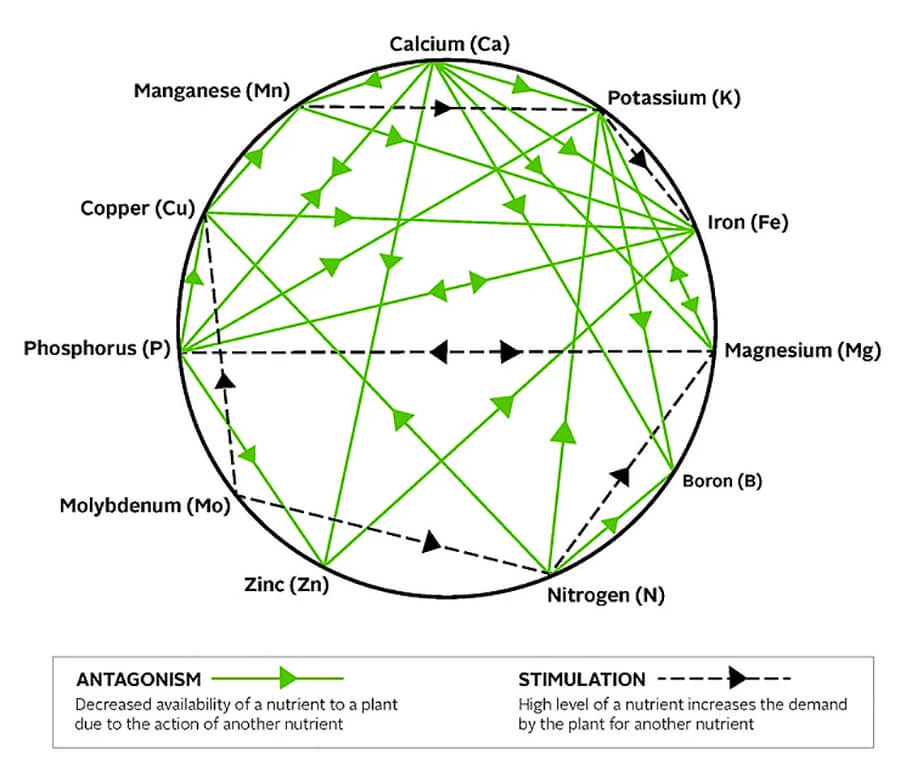
In this version of the Mulders Chart you can see how different nutrients compete or confuse plants. Nitrogen can cancel the effect of Potassium. This is why the fertilizer I use to encourage blooming doesn’t contain much nitrogen.
I have also found it difficult to source all the raw materials Steve loves so only one piece of information he told me really sticks and is the first specific thing to remember: If your plant leaves are healthy and shiny, the plant is receiving the ideal nutrients it needs. I use this concept even when shopping for groceries. Only shiny leaved, dark green spinach or lettuce comes home when I am out buying greens and my own home-grown goal is glossy spinach. Listen to my interview with Steven HERE.

Strawberry plant is healthy and the leaves are glossy – when plants look like this fertility levels are close to ideal.
Because some people learn by listening and others want to read and see pictures, I have included photos from my garden and gardens I have visited in this blog post about fertilizers.

The two books I referred to in this blog post – one is fifty years old and one is 10 years, but lets face it soil doesn’t change that fast!
Role of Fertilizer Elements:
Magnesium is part of the Chlorophyll molecule so the most common deficiency symptom when plants lack magnesium is inter-veinal chlorosis on older leaves (symptoms start with dark green veins and light green space between.) In spruce or fir trees, needle growth is stunted or leaves turn yellow at tips when Magnesium is unavailable but this is only seen in greenhouses because forests feed their young. (High levels of K- or potassium – will also aggravate the problem by reducing Mg uptake – see mUlders details above.)

Magnesium deficiency in this lemon leaf may be a sign of high potassium (K) or low magnesium. I have started watering my lemon plants quarterly with Epson salts in water because this reduces the pH and allows the magnesium from th eepson salts to be more available to my citrus plants.
Iron, similar to Magnesium, is important to photosynthesis. And it is also linked to inter-veinal chlorosis, but especially when soils are naturally high in pH – like the naturally high pH clay soils in my Calgary location.

Amur maple growing in high pH soil shows signs of Iron deficiency. But is iron actually lacking? Probably not .The plant just can’t access the iron because of the high soil pH.
Fertilizers, like those containing Nitrogen, promote rich, lush growth and a dark green colour. I have noticed that gardeners growing in poor outdoor soils or in pots often have stunted or dwarf plants. At very least, container-grown plants have leaves lacking in rich green colour.

This zucchini leaf is nice and green after fertilizing with a nitrogen rich fertilizer because nitrogen is very mobile and can move to where plants need it. Anything grown in containers usually needs extra fertilizer because potting mixes are notoriously low in nutrients.

These pale green zucchini leaves were grown in a container where they gradually used up all the available nitrogen in the potting mix. I fertilized them and they turned dark green (photo above) because nitrogen is very mobile in plants. Low nitrogen is an easy problem to fix in plants that are small or pale.
Other elements, like Phosphorus, “help develop strong root systems (especially on root plants like beets) but also help improve flowers, seed development early maturity and a normal, healthy green colour.” A symptom of red in leaves often means phosphorus deficiency – possibly because leaves are not able to pull up nutrients from limited roots or because soil is acidic and this means it is not as available?

Young lemon cutting with limited roots. There may have been more roots if I had fertilized the pots at some point after taking the cuttings.

Severe phosphorus deficiency in my sandy acidic soil on Vancouver Island inside my greenhouse. I guess I should have fertilized this tomato more! Yikes

A minor phosphorus deficiency shows as very pale red parts on these raspberry leaves probably due to acidic soil in my Qualicum Beach garden even though I did add Aglime almost every year to neutralize the soil and top-dressed plants with compost every spring. Heavy rains in winter lead to acidity and leaching of available nutrients held loosely in sandy soils.
Potassium has a balancing effect and promotes strong stems, good roots and resistance to disease. It is also essential for translocation of sugars in plants.

Strawberry leaf isglossy indicating it is healthy.

Shiny leaves on my Romaine lettuce indicate they are nutrient packed and healthy.
Sulfur, like nitrogen, promotes dark green leaves, and a lack of it may result in yellow leaves or the failure of some plants to set seed. Sulfur may also be of particular importance to healthy cole crops like broccoli, cauliflower and kale.
Calcium is a major component of cell walls (may also be deficient in overly acidic soils) and is related to nutrient transport within a plant. Anything interrupting normal calcium movement (such as uneven water supply) will result in calcium deficiencies even if there is enough Calcium added via fertilizer or showing up in soil tests.

When the “blossom” end (not the stem end) of a tomato turns brown and become sunken this is often blamed on a shortage of Calcium. But it might be due to sloppy, uneven watering. When there are sudden hot conditions or when the gardener is away (ie for the weekend) and the soil goes dry, calcium shortages show up as sunken brown blossom ends. Some types of tomato are more susceptible than others.
Optimum nutrition puts a a glossy coat on leaves, making them more resilient to disease, insects and trouble of all kinds and this is the all important Point 2 I want you to remember. Natural soils with a high clay content hang on to nutrients as ions, and release these ions as plants need them. This is why if plants are grown only in potting mix, compost or bagged “soils” instead of natural clay-containing soils there is a real need to add fertilizer as soil nutrients become “used up” (otherwise you will not get glossy leaves.)
Bringing Plants into Bloom
It is one thing to have a lemon as a houseplant in a northern garden. It is a completely different thing to have your healthy lemon houseplant bloom and produce lemons. And this is where specialty fertilizers come in. They are not a big dump of equal parts Nitrogen, Phosphorus and Potassium. The are specialized to balance the nutrients and trigger bloom. I use a water soluble fertilizer (1-25-35) with added kelp and micro-nutirents and when I am ready to stimulate blooms in spring when higher light levels allow it, I use it once or at most twice.

Keeping a houseplant alive is one thing. Bringing a lemon into bloom in your northern home or garden is another. Use the right fertilizer and making sure plants receive enough light so their energy is high!
Role of Soils
“Poor gardens and lawns are usually due to poor physical or chemical condition of the soil, or both. Of course, even with the best soils, gardens and lawns still require adequate, but not too much, moisture if they are to grow well.” From Soils and Fertilizers for Gardens and Lawns by C.F. Bentley and J. A. Robertson, Department of Soil Science, The University of Alberta, 1977
Notice Drs. Bentley and Robertson don’t mention fertilizer on page one of their book about soils and fertilizer. They mention soil physical and chemical composition. And water. And although their book is almost 50 years old, and only 48 pages long, it was an essential primer when I was a student and is still relevant from the view of soil physical and chemical condition.
One factor affecting soil chemistry is soil pH. Soils high (basic) or low (acidic) in pH will affect specific organisms in the soil and this affects nutrient availability. According to another excellent book Elements of the Nature and Properties of Soils high calcium and near neutral pH generally result in the largest, most diverse bacterial populations. Also, the authors’ stress soil fauna and flora as indispensable to plant productivity. One example they give is that nitrates ( a necessary form of plant-available nitrogen) are present in the soil due to microorganisms transforming them from ammonia ( a frequent ingredient in fertilizer that is inaccessible to plants.) Likewise phosphorus, sulfur, Iron and manganese are more available because of microbial activity so the authors advise you keep your soils at a neutral pH.
Soils in every garden will differ as the natural soils in the area differ but the importance of soil management to keep the soil neutral is paramount. Adding organic matter and other amendments affects soil chemistry and soil physical condition so be careful what you add when trying to improve soils.
Another very important effect on microorganisms is water. If there is stagnant or sitting water, available oxygen levels are lower and the natural levels of beneficial microorganisms are also lower. Again, the amount of organic matter in natural soil affects the drainage of the soil and the air spaces retained in the soil.

Excellent friable soil that drains well and is neutral in pH is the goal. Sadly, all native soils are not so perfect, which means fertilizers or the elements they contain are not always accessible to plants.
Role of Soil Amendments
Peat moss, compost, worm castings, perlite, humic acids, bone meal, eggshells, gypsum and biochar are all amendments that have various effects on the soil, some chemical and some physical.
Most people know, for instance, that compost is slightly acidic and is an excellent addition to clay soils, especially high pH clay soils because it makes their physical structure easier to work and also changes their chemical structure as it neutralizes pH and encourages microorganisms to multiply and convert nutrients into available particles for plants.

Sifting my own home-made compost before adding it to my soil involves a bit of elbow grease to remove the big parts that are slower to break down. In my sandy soil on the west coast, compost helped my soil by holding nutrients. In my current clay soil in Alberta, compost and the microorganisms it brings helps to soften the soil, making it drain faster and easier to work.
Gypsum is sold as a soil conditioner. According to Brady and Weil in their book “Elements of the Nature and Properties of Soils”, Gypsum (Calcium sulphate) provides enough ions to flocculate soil and inhibit surface crusting.This allows water to enter soils better and erode less. It also allows greater root penetration where there is an impassible subsoil layer. Bacteria may do the same thing but gardeners always demand a faster answer than just building soil biology!
On the west coast, Biochar increased my CEC (cation-exchange-capacity) and made me feel good because nothing else seemed to help my sandy soil hold more ions and dole them out to plant roots.
Peat moss used to be an annual addition to my clay soils in Edmonton, but we now know it is a limited natural product so I only use it to start seedlings, not to amend soil. Perlite is an expensive amendment and I limit it to houseplants, like citrus, that need better drainage.
I am a huge believer in worm castings and both make my own and buy bags of castings from local producers. It adds a special something to soil the way compost does and also adds microbes so soil is softer over time and easier to work. But adding the beneficial bugs does not make a meaningful addition of fertilizer to really poor or nutrient deficient soil.

Worms I have raised because it is fun, builds my soil and adds beneficial microbes. But it is a mess, takes space in my basement and adds tons of work to my schedule so sometimes I just buy the finished “worm poop” or castings.
Bone meal and egg shells don’t make the cut in my garden because there are better ways to add Calcium or phosphorus to boost plants. But I do save shells, blend them when dry and add the powdered shells to my compost heap.
I buy but never remember to use humic acids. Brady and Weil also mention humic acids, marketed for their soil conditioning effects when incorporated at low rates, but in their 3rd edition they had no results to share on effectiveness even though many studies had been done.
What Were Those Two Points Again?
Fine tuning outdoor, natural soils by adding organic matter, watering as needed, boosting beneficial natural soil microbes with worm castings, modifying pH with organic matter added or using Aglime (instead of dolomitic lime) if pH needs upping is important.
In other words, with proper soil management few, if any, additional fertilizers will be needed to grow plants in rich natural soils.

Many “secret” products or ingredients are actually kelp (see back side of this label below). So watch the price. So many forms of kelp are available and I would hate it if you paid for water with just a bit of kelp and in fact I prefer the dry forms or to buy fertilizer with kelp included. Just add water is an easier idea.

The label side of the Thrive Alive product shows it is really is only kelp (with vitamin B1) Kelp is a good thing, but I prefer the dry over the liquid forms and I mix it as I go.)
For planters or in any potted plant such as a raised bed for vegetables, the conditions are so different from natural soils that fertilizers are most definitely needed. And when you add fertilizer don’t forget to add a source of Kelp when you can because “(when) you add a little bit of kelp (along with fertilizer) it seems like the plant’s able to use it’s food and combat the stresses in its environment a lot better than (with) nutrition alone,” says Dr. Trobacher in my podcast interview with him. And he’s the doctor, not me.

Kelp is available as a liquid and as a dry product in more than one type of fertilizer and it is also sold as a single item in products such as Thrive Alive. Mix a bit (according to label instructions) when adding other fertilizer for best effect!
Just to repeat the two things to look for in your own garden:
#1 Low Nutrition often means dull, small, pest pressured plants.
#2 Optimal Nutrition means larger, glossy plants with low pest pressure.

This kale, at end of season illustrates how plants look when fertility levels are low…. . Plants and leaves are small and insect pressure is high. Would you serve this kale for supper?

This kale, grown in my garden close to the deficient kale shown above has perfect nutrition even though it was not fertilized this summer. Plants show optimum fertility with their shiny, large leaves and low insect pressure.